To the UOW Executive-Dean of the Faculty Science, Medicine and Health
Newsletter 151 Dr. Anthea Rhodes Providing False Information on Vaccines (http://us8.campaign-archive1.com/?u=f20605fde3732e41929f4a3f2&id=d553b59c83&e=fec8337d3c)I am also wondering why Professor Heather Yeatman has been allowed to present her personal opinion of vaccine safety on the University of Wollongong website?
Professor Yeatman is a specialist in nutrition and is in the Faculty of Health and Society not Science, Medicine and Health.
The information she is providing is not supported by the medical literature and Heather Yeatman and the UOW academics supporting this statement have not done an in-depth investigation of vaccination science.I would like to request again that in your role as a toxicologist and in supporting Health Yeatman’s claims on immunisation on the UOW website, that you inform the public that it is safe to add the following chemicals into the tissues of newborn developing infants (https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/B/excipient-table-2.pdf ). Whilst you are not required to answer our questions this is an important health issue and new legislation is being passed that will directly affect children’s health. It is a serious situation if you are promoting false information on vaccination on a university website.I hope that your concern for children’s health will ensure that you reply to this email that is copied to the community. I look forward to your response and I will publish this email and your response in my global Newsletter 152.Kind regards,Dr. Judy Wilyman
Newsletter 150 Fake News on Vaccines in the Mainstream Media
10 March 2017
Are parents getting faith-based information on vaccines from politicians, religious leaders and journalists or are they receiving the evidence-based information that is being provided to pharmacists in the pharmacists newsletter – Are Vaccines Safe?
The people questioning vaccines include doctors from the International Medical Council on Vaccination, the World Association for Vaccine Education and American Physicians and Surgeons who have published an article titled “Combining Childhood Vaccines at One Visit is not Safe“.
When the mainstream media presents false information on health issues by unqualified speakers, public health is put at risk. It also produces a situation where everyone’s opinion is equally valuable and this nullifies the integrity of university research. It produces a society that believes in a “flat earth theory”.
Currently the Australian media is presenting opinions on my PhD research from people who are not qualified to speak on the assessment of university research. False claims about my research have been made by reporters such as Kylar Loussikian (News Ltd) and also by activist, John Cunningham (a medical practitioner and administrator of the SAVN pro-vaccine lobby group). The opinions of these individuals are influencing public behaviour and resulting in politicians implementing dangerous legislation that is not in the public interest. For example, the No Jab No Pay Policy which Australian Public health officials would not debate.
Although I wrote an open letter to many journalists who have provided misleading information about my research, I have not received a reply from either the journalists or the University of Wollongong. Journalists are continuing to present this false information in articles, such as the one by Stephanie Peatling and Amy Remeikis, in the Sydney Morning Herald. This article represents propaganda and there is a lack of accountability for reporters presenting biased and false health information to the public. Here is a list of the false and misleading information provided by these SMH reporters:
1) It is organised pro-vaccine lobby groups (many funded by pharmaceutical companies) who are using social media to “muddy the evidence” and confuse the public with disinformation. Not parents who are concerned about children’s health.
2) Informed consent to all medical interventions depends upon “choice”, “education” and “alternatives”. These words should not be dismissed by these journalists as unimportant or irrelevant.
3) It is pro-vaccine lobby groups who are assisting parents such as Mrs. Riley (2016) and Mrs.McCaffery (2009) to present “emotive and personal stories” to promote the whooping cough vaccine to the public. When academics point out that this is anecdotal evidence that is not used to develop policies on vaccines the pro-vaccine lobby groups use the media and social media to claim “parents are attacking grieving parents“ or harassing them. The Australian Skeptic Inc lobby group that has a powerful voice in the Australian media also provided a financial award to the McCaffery’s in 2009 for their involvement in promoting the whooping cough vaccine to the public (this is not to suggest that the McCaffery’s were paid to promote the vaccine).
4) The pro-vaccine lobby groups, for example, Stop the Australian Vaccination Network (SAVN) have set up an Anti-Vax Wall of Shame, on facebook to tarnish the reputations of academics and professionals who provide the risks of vaccines that are listed in the medical literature. The blogs of these lobby groups, such as the deceptively titled “ReasonableHank” use abuse and ridicule to provide disinformation to the public on vaccines. These bloggers are associated with the Australian Skeptics Inc lobby group and most, such as Peter Tierney who runs ReasonableHank, do not have qualifications in vaccination science or policy. The SAVN lobby group suppresses academic debate with false and misleading information with the assistance of these bloggers.
5) It is the funded pro-vaccine lobby groups who are using “guerrilla social media techniques” to influence public behaviour on this issue – not the unfunded parents asking valid questions about the safety of vaccines for their children.
6) It is the influence of pharmaceutical companies in the mainstream media, government and universities that is skewing the medical literature to promote industry interests in government vaccination policies. And this is assisted by lobby groups being able to use the university procedures to present false and misleading information about academic research.
7) These SMH journalists are repeating the false claim that the link between vaccines and autism has been discredited.
8) It is the organised pro-vaccine lobby groups that are “quickly mobilised” and ringing talkback radio stations to air their views not the unfunded parents asking genuine questions.
8) These lobby group activists are falsely framing the debate and ignoring the bereaved parents of vaccine-injured children and others who know their life-time chronic illnesses are preventable if unnecessary vaccines are removed from the schedule.
9) I will echo the comments by Professor Brian Owler, former president of the Australian Medical Association, and say that the consequences of not addressing the false claims made by the pro-vaccine, industry supported lobby groups is too dangerous.
10) Parents should not rely on the biased claims being made by news reporters, politicians, and industry funded lobby groups who are influencing media content and the internet. They should investigate for themselves using independent academic literature that is addressing the concerns regarding all childhood illnesses and vaccine injuries – not just those caused by infectious diseases.
Public Health is at risk until transparency in policy decisions is restored and until public debates of the scientific literature are not being suppressed by powerful lobby groups using the media and university processes. Here is a letter to the Australian Prime Minister, Malcolm Turnbull, by Elizabeth Hart, requesting an urgent review of Australia’s vaccination policies and a reassessment of the health outcomes of the No Jab No Paylegislation.
Dr. Judy Wilyman
The Science and Politics of Australian Vaccination Policies
Vaccination Decisions
2. Vaccine Science is Not Settled: A Critical Review of the Literature
A White Paper by Brian S. Hooker, PhD, PE,
and the Alliance for Natural Health USA
Purpose
Since the modern inception of public mistrust in vaccines in the late 1990s, in the wake of a since-retracted study published in The Lancet by Andrew Wakefield in 1998,[1] a handful of sporadic and hastily completed epidemiological studies have been used by the media and various government agencies as evidence that vaccines are safe. As Mark Twain quipped, “Facts are stubborn, but statistics are more pliable.” There are many ways in which data is chosen, manipulated, and compared, and the average person with only a basic knowledge of statistical methods must take the word of the author that the results are “proof.” Important policy decisions and laws are being made, and public debate is being suppressed, based on the unexamined assumption that the science on vaccine safety has been settled. The Alliance for Natural Health USA (ANH-USA) and Dr. Brian Hooker have teamed up to rigorously scrutinize twelve of the papers most often cited as proof positive that vaccines are safe. Each review presents the study’s overview and findings, along with an analysis of the findings, and a final statement of the authors’ conflicts of interest.
Summary of Findings
Generally speaking, each study was conducted by researchers connected to government immunization offices or drug companies, thereby giving them a vested interest to “prove” that vaccines are universally safe. Other problems were identified: poor sample choice, data inconsistencies, unproven assumptions, subjective dismissals of data contrary to conclusions, faulty comparisons, unreliable methods, and unsupported conclusions. Given these flaws, one cannot take this research as proof positive that vaccines are as universally safe for children as pharmaceutical companies and government agencies claim. We do not posit that vaccines are a cause of autism—but we point out that these studies cannot allay all concerns, given the following issues.
No evidence for measles, mumps, and rubella vaccine-associated inflammatory bowel disease or autism in a 14-year prospective study (1998)[2]
Authors: Peltola H, Patja A, Leinikki P, Valle M, Davidkin I, Paunio M.
Study overview: This study was conducted in response to a previous study conducted by Andrew Wakefield et al. in 1998 which suggested a link between the measles, mumps, and rubella vaccine, and chronic inflammatory bowel disease related to autism.[3] Peltola et al. reviewed data from the US Vaccine Adverse Event Reporting System (VAERS) to claim that GI symptoms were rare after the measles, mumps, and rubella (MMR) vaccine. Their findings come from data that showed 31 individuals complaining of gastrointestinal symptoms after receiving the MMR vaccine out of 3 million vaccines administered in the years studied. This study is often cited as evidence that the MMR vaccine is safe.
Study’s flaws: The VAERS database is a federally funded surveillance system based on self-reporting, meaning that only those with the wherewithal to report their adverse event will do so.[4] The VAERS system itself states: “‘Underreporting’ is one of the main limitations of passive surveillance systems, including VAERS. The term ‘underreporting’ refers to the fact that VAERS receives reports for only a small fraction of actual adverse events.”[5] By VAERS’ own accounts, many more than 31 may have experienced symptoms without reporting them. This type of study is inherently limited by relying solely on VAERS data. It cannot rule out that a larger population experienced GI systems after receiving the vaccine, nor can it rule out that a specific subpopulation could be vulnerable to the MMR vaccine, similar to the children evaluated in the 1998 Lancet study.
Conflicts of interest: This study was funded by Merck, which makes the current formulation (MMR-II) of the measles, mumps, and rubella vaccine, and stands to profit from results that disprove any dangers.
A population-based study of measles, mumps, and rubella vaccination and autism (2002)[6]
Authors: Madsen KM, Hviid A., Vestergaard M., Schendel D, Wohlfahrt J, Thorsen, P.
Study overview: Admitting that previous studies disproving the link between MMR vaccine and autism were limited by design, and responding to the World Health Organization’s call for further study, the authors sought a larger data sample to increase the statistical analysis capabilities. They conducted a retrospective study on children born in Denmark between 1991 and 1998, after the MMR vaccine was approved for use in Denmark in 1987. They compared the cohort of unvaccinated children to the nearly half a million children who received the vaccine, and found an insignificant relative risk of autism and autism spectrum disorders in the vaccinated population.
Study’s flaws: The data presented by the researchers in their findings is inconsistent, calling into question their entire methodology. Table 1 reports 269 vaccinated children with autistic disorder and 47 unvaccinated children with autistic disorder. Table 2 reports 263 vaccinated children with autistic disorder and 53 unvaccinated children with autistic disorder. Using one set of numbers instead of the other changes the results from slightly less than a relative risk of 1 to slightly more, when the vaccinated cohort is compared to the unvaccinated. That their findings are based on inconsistent data should be enough for credible reporting on vaccinations to not cite the study. Also, the authors themselves admit that this type of study does not rule out that a subpopulation could be vulnerable to the MMR vaccine as presented in the 1998 Lancet study.
Conflicts of interest: This study was funded by the National Immunization Program (NIP) at the US Centers for Disease Control and Prevention (CDC), which had a vested financial interest in increased uptake of the MMR vaccine, as it directly bought the vaccine from Merck and distributed it for reimbursement to the states’ public health departments. Also, one of the co-authors of the study, Dr. Diana Schendel, was a CDC employee at the time of publication. In addition, three of the co-authors (Dr. Mads Melbye, Mr. Jan Wohlfahrt, and Mr. Anders Hviid) were employees of Staten Serum Institut, a for-profit company that manufactures and distributes vaccines in Denmark.
Do children who become autistic consult more often after MMR vaccination? (2001)[7]
Authors: DeWilde S, Carey IM, Richards N, Hilton SR, Cook DG
Study overview: The researchers posited that if the MMR vaccine was causing autism, then children with symptoms would have a significant increase in general practitioner doctor visits post-immunization. Data from England during the years 1989–2000 was used to compare visits to the primary care provider of autistic children versus visits by non-autistic children within six months of receiving the MMR vaccine. They found no significant increase in doctor visits within this six-month time period.
Study’s flaws: The premise of this paper is based on the assumption that if the MMR causes autism, then the number of general practitioner visits would increase for autistic children after the administration of their first MMR vaccine. This does not take into account the total number of practitioner visits, which would include specialist visits. It stands to reason that most general practitioners would not see autistic children at a greater frequency but instead would make referrals to specialists, such as behaviorists, neurologists and gastroenterologists, who would be sought out to attend to the symptoms of an adverse reaction. The data also does not show how many children may have sought treatment for their symptoms outside of the narrow 6-month window studied. Finally, there were no autism specialists among the authors who could speak to how and when autism presents and is diagnosed.
Conflicts of interest: DG Cook, one of the paper’s authors, worked as a professor in the Department of Public Health Sciences at St. George’s Hospital Medical School. Public health practitioners are generally trained to accept vaccination policies without question and would not serve as objective researchers.
Measles, mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: population study (2002)[8]
Authors: Taylor B, Miller E, Lingam R, Andrews N, Simmons A, Stowe J.
Study overview: This paper focused on autism cases identified in five districts of London, England, for children born nine years prior to and nine years after the introduction of the MMR vaccine in the UK in 1988. The authors hypothesized that if autism were related to the MMR vaccine, then there would be a corresponding increase in the number of autism cases after 1988 as well as rising rates of regressive autism (in which a child with typical developmental levels suddenly begins to lose speech and social skills and is subsequently diagnosed with autism). Additionally, the researchers sought to determine if the MMR vaccine was linked to increased incidence of bowel disorders among children with autism, perhaps indicating a “new variant” form of autism. The authors concluded that there was no “new variant” form of autism characterized by regressive autism and bowel disorders, and further, that there was no link between the MMR vaccine and autism.
Study’s flaws: The authors claim that there is no statistical evidence of any link between the MMR vaccine and autism, yet the raw data suggests otherwise. Consider their report of the number of children whose parents reported symptoms: only 29% of parents reported an incidence of autism, regressive autism, or bowel disorder before the child was vaccinated, as opposed to 58% of parents who reported symptoms after the vaccine. Only 13% of reported cases occurred in unvaccinated children. The authors caution in their conclusion that some parents may have changed the age at which they noticed symptoms after the Wakefield study made news, but this is complete conjecture on their part, and certainly does not explain such a large differential. Their own data suggests that more children overall were “regressing” after the MMR vaccine rather than before.
Conflicts of interest: Two of the study’s authors, Nick Andrews and Elizabeth Miller, are a part of the UK’s immunization division in the Public Health Laboratory Service, Communicable Disease Surveillance Center. This particular division is in charge of immunization uptake in the UK as well as vaccine safety.
No evidence for a new variant of measles-mumps-rubella-induced autism (2001)[9]
Authors: Fombonne E, Chakrabarti S.
Study overview: This study sought evidence of a new form of autism characterized by developmental regression (in which children who seemed to be achieving developmental milestones began losing ground and were subsequently diagnosed with some form of autism) and bowel disorders in children, as posited by the Wakefield study. The authors contended that if such a new form existed, then they should find statistical evidence in at least one of the following ways:
- there should be a higher incidence of this type of disorder in the general population;
- first parental concern should be closer to mean vaccination age in vaccinated vs. unvaccinated children;
- there should be an increase in regression of autistic children in vaccinated vs. unvaccinated children;
- autistic children with regression would be clustered around age at vaccination;
- autistic children with regression would have distinct symptoms with similar severity; or
- regression autism would be associated with gastrointestinal and/or bowel disorders.
The authors used three sample sets to identify statistical relationships between the administration of the MMR vaccine and evidence of any of the above listed indicators. The first sample was a purposive sample comprised of children who had been identified with pervasive developmental disorders through a UK survey in Staffordshire. The sample consisted of 96 children who were born between 1992 and 1995, all but one of whom had received the MMR vaccine. The second sample was a convenience sample, in which the subjects volunteered or were chosen to take part in the study, specifically culled from the practice of the paper’s first author, Dr. Eric Fombonne. This sample was also comprised of children who received the MMR vaccine after 1988 (when the MMR vaccine was introduced), including 68 children born between 1987 and 1996 who had a confirmed diagnosis of Pervasive Developmental Disorder (PDD). The third sample was also a convenience sample, of 99 individuals with autism who were born before the introduction of the MMR vaccine.
The authors claim that their analysis found no statistical evidence to support any of the above claims, thereby establishing there was no new form of autism and no link to the MMR vaccine.
Study’s flaws: Comparing purposive samples to convenience samples is not a valid research method and therefore should not be relied upon to make inferences regarding the MMR vaccine and autism. The authors also do not list the source of the third convenience study, further calling into question the validity of the sample.
Conflicts of interest: After this paper was published, lead author Dr. Fombonne violated scientific ethics at his post at Montreal Children’s Hospital and McGill University when he improperly used samples derived from autistic children for another study unauthorized by their parents. Dr. Fombonne has also testified as an expert witness for vaccine manufacturers and against families with reportedly vaccine-injured children, both in civil courts and the National Vaccine Injury Compensation Program for the US.
Time trends in autism and in MMR immunization coverage in California (2001)[10]
Authors: Dales L, Hammer SJ, Smith NJ
Study overview: The goal of the study was to find a correlation between increased autism rates and increased MMR vaccination rates in the general population. The authors used an ecological study, which looks at the whole population for incidences of a disease rather than examining individual cases of autism. The data came from the state of California between 1980 and 1994, using kindergarten enrollment to determine the number of children vaccinated between 17 and 24 months of age, and comparing it to the number of children born during these years who received services from the California Department of Developmental Services after an autism diagnosis. Their analysis shows that while autism rates in California increased in this time period by 373% (from 44 cases per 100,000 live births to 208 cases per 100,000 live births), the relative uptake of the MMR vaccine by 24 months increased by only 14%, leading to their claim that the rise in autism rates cannot be account for by the relative rise in MMR vaccinations.
Study’s flaws: A relationship between the MMR vaccine and autism cannot be ruled out at an individual level based on this comparison of overall rates alone. Increases in the use of the developmental centers may be bolstered, as the authors acknowledged, by increased availability of the centers and increased public awareness of autism. Further, in a rebuttal, Edwards and Baltzan remapped the data and found that the age at immunization was actually trending younger than 17 months between 1981 and 1993,[11] and that the original comparison plots were vertically compressed—and once adjusted actually suggest a correlation, if not a causation.
Conflicts of interest: The lead author of this publication, Dr. Loring Dales, was the head of the immunization branch of the California Department of Health Services at the time of publication of this paper. Dr. Dales then had a responsibility to maintain vaccine uptake (including the MMR vaccine) in the state of California.
Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: a time trend analysis (2001)[12]
Authors: Kaye JA, del Mar Melero-Montes M, Jick H.
Study’s overview: This study was a time trend analysis, where researchers look to see if one variable increases or decreases in tandem with another factor over a period of time. The researchers looked at the rate of increase of autism diagnoses among children between 1988 and 1999, and then specifically looked at boys born between 1988 and 1993, and the rate of MMR vaccine administration (uptake) during the same time periods. The authors claimed that there was no correlation despite a marked increase in autism incidence with time, because trends in MMR uptake remained unchanged.
Study flaws: Instead of defining autism incidence based on when the child was born, the study authors instead defined autism incidence based on diagnosis rate during diagnosis year, with the denominator corrected for age of diagnosis. This method gives a bias towards autism diagnosed at younger ages and is not a true measure of autism incidence. Therefore, this section of the analysis is essentially meaningless in determining true autism incidence versus MMR uptake. The rate of MMR uptake remained steady at over 95% throughout the birth cohorts while the autism incidence (as measured by the researchers) increased sevenfold. MMR uptake could not possibly proportionally increase as it was already a high 95%, so this type of temporal study sheds no light—it was de facto “proof” by design.
Conflicts of interest: The authors of this paper were residents at the Boston Collaborative Drug Surveillance Program at Boston University School of Medicine. This program is supported in part by grants from AstraZeneca, Berlex Laboratories, Boehringer Ingelheim Pharmaceuticals, Boots Healthcare International, Bristol-Myers Squibb Pharmaceutical Research Institute, GlaxoSmithKline, Hoffmann-La Roche, Janssen Pharmaceutica Products, RW Johnson Pharmaceutical Research Institute, McNeil Consumer Products, and Novartis Farmaceutica. Funding for their residency program is directly tied to those profiting from the vaccine companies their claims protect.
MMR and autism: further evidence against a causal association (2001)[13]
Authors: Farrington CP, Miller E, Taylor B.
Study overview: The authors of this study investigated time trends of autism diagnoses in a case series involving 357 UK children diagnosed with autism. They looked at temporal relationships, that is, how much time elapsed between administration of the MMR vaccine and a subsequent autism diagnosis. Farrington et al. looked at two different time spans—36 months and 60 months, respectively—between the MMR vaccine and the first reported parental concern, as a previous study had only looked for a closer temporal relationship. Additionally they looked at 24 months as an interval between the MMR vaccine and onset of regressive autism. They claim their results showed no correlation as the incidence of autism increased while the MMR uptake remained steady. Importantly, the World Health Organization has used these findings to support their immunization policy.[14]
Study’s flaws: Despite the authors’ claims, the data presented as evidence in the paper instead seems to actually confirm a temporal relationship between the MMR vaccine and the age of first diagnosis. This is best shown using their own data charts. Figure 1 includes the age of first diagnosis for 64 unvaccinated children with autism and shows significantly more “scatter,” as compared to Figure 2, for 231 children receiving a single dose of the MMR vaccine, where diagnoses tend to cluster between 0 and 30 months post-vaccination.
Fig. 1. Distribution of age at autism diagnosis (in months) of 64 unvaccinated children with autism.
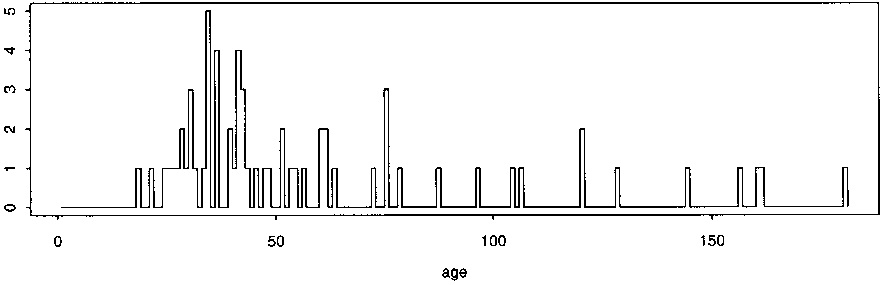
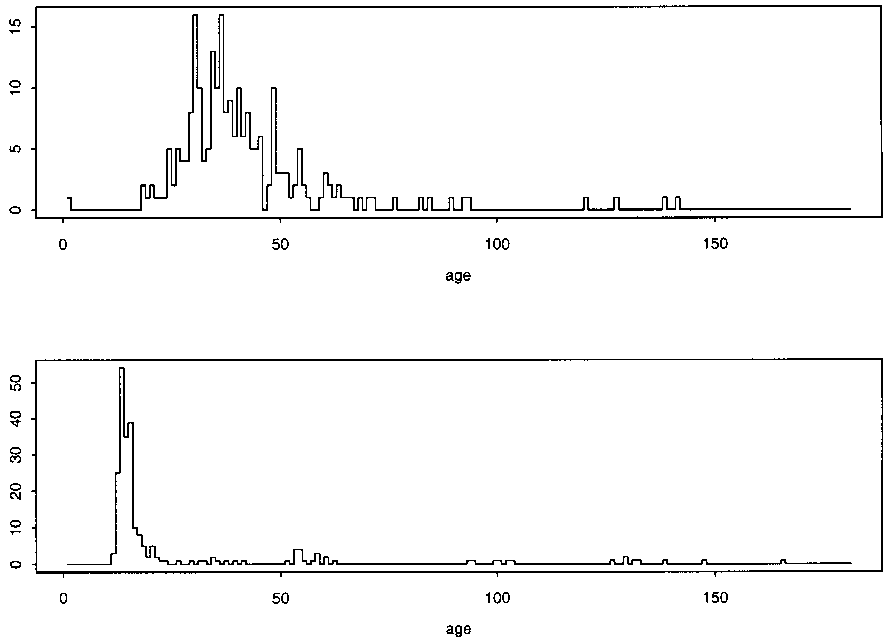
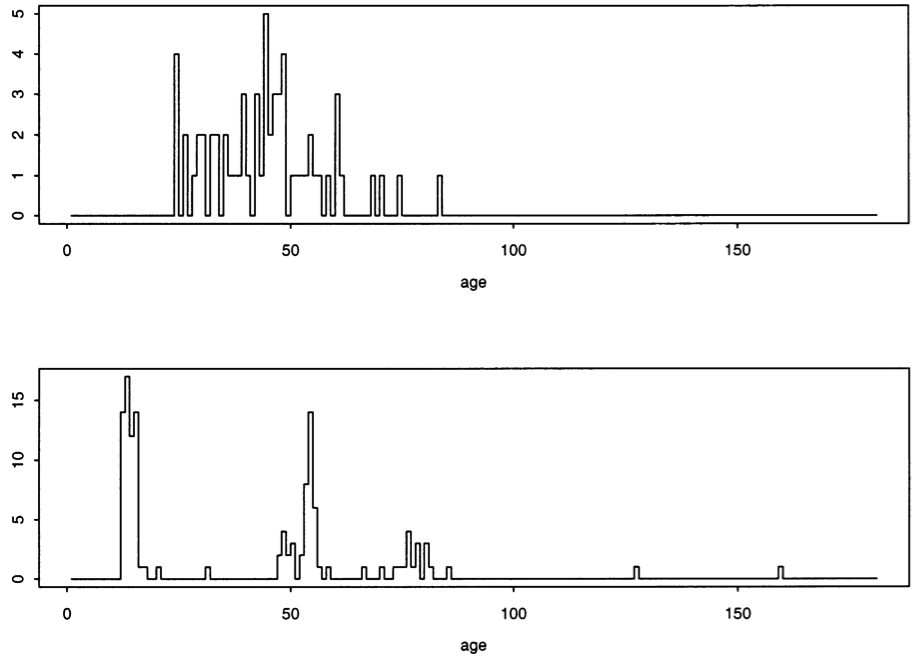
Fig. 2. Distribution of (top) age at autism diagnosis and (bottom) age at vaccination (in months), of 231 children with autism who received a single dose of MMR vaccine.

Figure 3, of 62 children receiving two doses of the MMR vaccine, also shows clustering between 0 and 30 months after administration of the first vaccine.

Fig. 3. Distribution of (top) age at autism diagnosis and (bottom) ages at vaccination (in months) of 62 children with autism who received two doses of MMR vaccine.
The study authors blame this phenomenon on time trends where cases post-MMR vaccine tend to be newer and cases without the MMR vaccine tend to be older, because newer cases would be subject to earlier diagnosis and screening. This statement is completely unsubstantiated in the paper, however, and does little to counter the clear clustering of autism diagnoses after vaccination.
Conflicts of interest: One of the authors, Dr. Elizabeth Miller, was a chief official at the Immunization Division of the UK’s Public Health Laboratory Service (PHLS) and possessed an institutional conflict of interest, given the PHLS’s stake in maintaining high vaccination rates in the UK.
Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association (1999)[15]
Authors: Taylor B, Miller E, Farrington CP, et al.
Study overview: This study provided a comparison of autism rates before and after the introduction of the MMR vaccine in the UK in 1988. Data showed a sharp increase in autism incidence in the early 1990s but did not appear to correlate with MMR vaccine uptake, which plateaued soon after introduction. According to the study authors, although most evidence did not show a temporal relationship between MMR vaccine timing and “autism onset,” there is compelling data for the age of first parental concern clustering between 0 and 6 months of administration of the MMR. The authors dismiss this finding by arguing that parents chose an age of first concern of 18 months simply because it was a round number.
Study’s flaws: The claim that parents chose an age of concern of 18 months because it was a “round number” is unsubstantiated and therefore not a valid explanation for the cluster of data that shows otherwise. Data findings should not be subjectively dismissed at the convenience of the researchers.
Conflicts of interest: Two of the study authors (Dr. Elizabeth Miller and Ms. Pauline Waight) are from the UK’s Immunisation Division, Public Health Laboratory Service Communicable Disease Surveillance Centre, which reflects institutional conflict of interest as this particular agency is responsible for increasing vaccine uptake within the UK population.
Neurologic disorders after measles, mumps, rubella vaccination (2002) [16]
Authors: Makela A, Nuorti JP, Peltola H.
Study overview: This study used hospital records to determine if there was a relationship between when the MMR vaccination and hospitalizations of children for three different diagnoses: encephalitis (within three months of MMR vaccine); aseptic meningitis (within three months of vaccination); and autism (throughout time period of analysis, which was 1982–86). Data was taken from hospital records in Finland. The authors claim that no temporal relationship exists between the MMR vaccine and any of the three diagnoses, including autism in a hospital setting. They report there was no clustering of hospitalizations for autism at any intervals following immunization.
Study’s flaws: This study actually clearly shows that peak rates of autism diagnoses occurred after the MMR vaccine was administered, especially in the 0 to 6 month window following vaccination. Additionally, the data is based on autism “hospitalization” visits, which is likely an under-ascertainment of actual autism cases, given that many cases are diagnosed in an outpatient setting.[17] Also, diagnoses in a hospital setting do not reflect the actual onset of symptoms, including regression, and the authors of this paper directly admit that this is a flaw in their analysis.
Conflicts of interest: Major MMR and MMR-II manufacturer Merck funded this study, and held a worldwide monopoly on these vaccines during the time of the research.
Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations (2006)[18]
Authors: Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D.
Study overview: This study focused on ecological methods—looking at the whole population for incidences of a disease rather than examining individual cases of autism—based on a population of students in a large school district in Montreal, Canada. The authors looked at the increases in developmental disorders which were identified in children by schools’ special needs teams, and compared them to (a) estimates of exposure to thimerosal (ethylmercury), which was phased out during the study, over the same time period, and (b) to changes in the MMR vaccination trends, including a switch to a two-dose MMR schedule during the study period. The authors concluded that there was no relationship between thimerosal exposure and increased developmental disorders. Further, they claimed that while the vaccination rate experienced a dip during the study period, there was no corresponding decrease in developmental disorders and also that there was no change in developmental disorders associated with the subsequent increase to a two-dose schedule.
Study’s flaws: The school district represented in this study accounts for only 14% of the total school population in Montreal but has the highest rates of pervasive developmental disorder (PDD, aka autism) diagnosis in the five total districts, and in some cases PDD rates were three times higher within this district. Also, the claim that the thimerosal exposure in the Canadian vaccine schedule starting in 1996 was “nil” is false. At this point in time, the thimerosal free HepB formulation had not yet been introduced (not until 2001) and the thimerosal containing “Penta” vaccine was administered, leading to a cumulative maximum dosage of 137.5 micrograms mercury. Essentially, this fact, along with the lack of actual vaccine records for cohort members, renders any time trend comparison between autism/ASD/PDD prevalence and thimerosal exposure useless.
Also, regarding the MMR vaccine uptake, the study authors use uptake as a metric of vaccine coverage, which was not done for the thimerosal analysis. The authors do not accurately reflect the effect of the introduction of two doses of the vaccine in 1996, which would essentially double the viral load administered to each individual. Finally, the errors associated with autism/ASD/PDD diagnoses essentially obviate any true indication of an upward trend, given that there is significant overlap in the 95% CI range at each year reported, including the endpoints of 1987 and 1998.
Conflicts of Interest: The lead author of this study, Dr. Eric Fombonne, has significant conflicts of interest, as he has many times represented vaccine manufacturers as an expert witness in thimerosal litigation, opposing the families of vaccine injured children.
Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan Atlanta (2004)
Authors: DeStefano F, Bhasin TK, Thompson WW, Yeargin-Allsopp M, Boyle C.
Study’s overview: The authors were looking for an association between the increased rates of autism in children and the MMR vaccine, both among the general population and in sub-groups of children. Researchers compared the vaccination schedules of autistic children from the general population to a control group of school-matched children who did not have autism. This study focused on children enrolled in public school within five districts in metropolitan Atlanta who received their first MMR vaccine at different time intervals. Autism cases were matched with three controls based on gender, birth year, and school of enrollment for a total sample of 2448 individuals. The researchers concluded that similar proportions of case children (those with autism) and control children (school-matched subjects) were vaccinated according to the recommended schedule and likewise with any of the subgroups also studied. One sub-group, boys ages 3 to 5 enrolled in special education services, had a rate of immunization earlier than their school-matched controls. However, the authors argue that this subgroup would have had the earlier immunization as a requirement for receiving school-based services and thus dismiss it as insignificant.
Study’s flaws: The study actually showed statistically significant relationships between autism incidence and MMR timing for all individuals considered in the study (OR: 1.49, 95% CI: 1.04–2.14) with a stronger association for males only (OR: 1.67, 95% CI: 1:10–2.53) for those individuals who received their first MMR vaccine prior to 36 months of age, with a reference of those individuals who received their MMR vaccine after 36 months of age. This is quite a compelling relationship, though the authors dismiss the effect, stating that children with autism would be enrolled in special education services which would require earlier vaccination with the MMR. This statement makes no sense, in light of the fact that the relationship is observed to come exclusively from boys in the cohort and not girls (no statistically significant relationship is seen when considering girls separately). There is no reason to assume that boys enrolled in special education services would receive earlier MMR vaccines than girls enrolled in the same programs.
Conflicts of interest: Two of these individuals were employees of the National Immunization Program of the US Centers for Disease Control and Prevention (CDC), which is charged to increase immunization rates in the US in order to prevent outbreaks of infectious diseases. Thus there was a propensity against finding a causal relationship between vaccines and vaccine adverse events, which is demonstrated clearly in the text of the paper by the authors’ faulty explanation when statistically significant relationships are observed. Dr. William Thompson, co-author of the paper and lead statistician with the CDC, has recently (August 2014) come forth with evidence of fraud committed specifically regarding this paper, and has launched a complaint against his co-authors regarding both the outcomes and the interpretations of the results of this study.
Conclusion
Studies undertaken by researchers with a vested interest either in immunization uptake or vaccine manufacture should be subjected to greater scrutiny. While the goal should always be the eradication of disease, both the profit motive and bureaucracy impede the necessary safeguards to public health. While the majority of a population may suffer no ill effects from vaccines, subgroups of the population may be at risk. After the public started questioning the safety of vaccines in the late nineties, public health agencies and drug manufacturers were quick to produce more than a dozen studies “proving” the safety of vaccines. Applying scientific scrutiny to some of these studies shows that they do not, beyond dispute, prove that vaccines are safe. Further independent, open-minded study is needed to prove the safety of vaccines for all subgroups of the population.
[1] Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351: 637 41.
Dr. Wakefield, together with twelve other colleagues, published a paper in the famed British journal The Lancet—a case study of twelve young patients who presented with a new type of bowel disorder, all of whom had autism and developmental delays. Parents reported that each of these children regressed into their condition after receiving the MMR vaccine (for measles, mumps, and rubella). It is important to note that this paper neither confirmed nor denied a connection between regressive autism and the MMR vaccine. However, it did indicate that it was an area worthy of further study.
[2] Peltola H, Patja A, Leinikki P, Valle M, Davidkin I, Paunio M. No evidence for measles, mumps, and rubella vaccine–associated inflammatory bowel disease or autism in a 14-year prospective study. Lancet 1998; 351:1327–8.
[3] Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351: 637 41.
[4] https://vaers.hhs.gov/data/index
[5] Ibid.
[6] Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002; 347:1477–82.
[7] DeWilde S, Carey IM, Richards N, Hilton SR, Cook DG. Do children who become autistic consult more often after MMR vaccination? Br J Gen Pract 2001; 51:226–7.
[8] Taylor B, Miller E, Lingam R, Andrews N, Simmons A, Stowe J. Measles, mumps, and rubella vaccination and bowel problems or developmental regression in children with autism: population study. BMJ 2002; 324: 393–6.
[9] Fombonne E, Chakrabarti S. No evidence for a new variant of measles, mumps, rubella–induced autism. Pediatrics 2001; 108:e58.
[10] Dales L, Hammer SJ, Smith NJ. Time trends in autism and in MMR immunization coverage in California. JAMA 2001; 285:1183–5.
[11] Michael Edwardes, PhD; Marc Baltzan, MD, FRCPC. MMR Immunization and Autism. JAMA June 13, 2001. http://jama.jamanetwork.com/issue.aspx?journalid=67&issueid=4787
[12] Kaye JA, del Mar Melero-Montes M, Jick H. Mumps, measles, and rubella vaccine and the incidence of autism recorded by general practitioners: a time trend analysis. BMJ 2001; 322:460–3.
[13] Farrington CP, Miller E, Taylor B. MMR and autism: further evidence against a causal association. Vaccine 2001; 19:3632–5.
[14] http://apps.who.int/iris/bitstream/10665/107553/1/e82636.pdf.
[15] Taylor B, Miller E, Farrington CP, et al. Autism and measles, mumps, and rubella vaccine: no epidemiological evidence for a causal association. Lancet 1999; 353:2026–9.
[16] Makela A, Nuorti JP, Peltola H. Neurologic disorders after measles, mumps, rubella vaccination. Pediatrics 2002; 110:957–63.
[17] Madsen K, Lauritsen M,Pedersen C, Thorsen P, Plesner A, Andersen P, Mortensen P. Thimerosal and occurrence of autism: negative ecological evidence from Danish population-based data. Pediatrics 2003; 112:604-606.
[18] Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics 2006; 118:e139–50.
3.Freda Birrrell– Sanevax-Censorship at NIH
Dear all,
In this world of vaccines and trying to raise awareness that the HPV vaccine is causing serious damage and harm to many worldwide is an uphill struggle, as many of you will know.
One eminent doctor in particular, Dr Sin Hang Lee, has put his head up above the parapet so many times and gets knocked down time and time again.
You ask what is he wanting to do, what is he wanting to say – the answer is simple he is wanting to tell the other side of the story and open up proper scientific debate on a very important subject.
But what happens is any of his well-constructed comments, his evidence supported by science claiming that a so-called expert’s published paper is not correct, does not give the true facts and Dr Lee challenges the author to an open and honest debate. In a democratic society, you would think that would happen and be totally acceptable but in the vaccine world that is not the case. Dr Lee’s views are discounted, his comments removed from the record and we are supposed to accept that and keep quiet!!
Well that is where they are so wrong – where we have voices we will speak out, where we are able to put pen to paper we will record our disgust at the inappropriate behaviour of those in high places who deny anyone the right to give their opinion. Read the attached SaneVax press release to get all of the facts.
What can you do, here is a suggestion and please anyone who wishes to comment on PubMed Commons’ apparent policy of censoring comments put forth in the spirit of encouraging high quality discussions of scientific issues can visit this page – https://www.ncbi.nlm.nih.gov/pubmedcommons/ On the lower right hand side at the very bottom of the page is a link to email them. It says “We value your feedback.” This will not take long to do, put in your letter of complaint and demand that Dr Lee’s original comments are all restored. Is Dr Mark Schiffman so frightened to open up honest debate with our Dr Lee or could it be that he knows what Dr Lee is saying is correct and therefore best to ignore and the problem will go away?
Not on your life!! So please take just a little time to give support to Dr Lee and to demand that the PubMed Commons policy of censoring comments from respected scientists is not democratic and does not belong, or should not belong, in any country where we have the right to free speech.
Link to the Press Release is here: http://sanevax.org/nih-marketing-hpv-vaccines-via-censorship/ – pdf copy attached, please share.
With best wishes
Freda Birrell, Secretary,SaneVax Inc
NIH: Marketing HPV vaccines via censorship?
By Norma Erickson
As an employee of the National Cancer Institute (NCI), part of the National Institutes of Health (NIH), it is certainly within Dr. Mark Schiffman’sjob description to write articles promoting human papillomavirus (HPV) vaccines.
After all, his employer owns patents on HPV vaccine production technologies and receives licensing fees from the sales of HPV vaccines.
The HPV vaccine, Gardasil, based largely on technology developed at NIH and produced by Merck & Co., was approved by the FDA in June 2006. As early as Feb 2007, an article was published in The NIH Record, titled, From Lab to Market: The HPV Vaccine proclaiming,
“Perhaps no other recent product on the market demonstrates successful health care technology transfer better than the HPV vaccine.”
What a great commercial success!
The NIH, funded by taxpayers, also maintains a forum for scientific discourse, called PubMed Commons which hopefully “will leverage the social power of the internet to encourage constructive criticism and high quality discussions of scientific issues that will both enhance understanding and provide new avenues of collaboration within the community”.
In December 2016, Dr. Schiffman and a few industry-paid consultants published an article titled “Carcinogenic human papillomavirus infection.” January 19, 2017, the eminent pathologist Dr. Sin Hang Lee commented via PubMed Commons stating: Schiffman and colleagues finally admitted in the end of the abstract that implementation of HPV vaccination and screening globally remains a challenge.
However, the authors did not present the whole truth required for a balanced analysis.
It took nearly a month for Dr. Mark Schiffman to respond to Dr. Lee’s public comment with reassurances that the efficacy and safety profile of Gardasil had been well established.
Five days later, Dr. Lee responded to Dr. Schiffman saying: Dr. Schiffman’s responses to my initial comment on the Primer needs rebuttal to point out its misleading and obfuscating statements.
Almost immediately, the discussion was effectively shut down by the removal of Dr. Lee’s comments.
Does this not seem like a gross violation of the public trust in an organization such as NIH which has promised to ‘encourage constructive criticism and high quality discussions of scientific issues’?
Is it not a serious conflict of interest for NIH moderators to remove Dr. Sin Hang Lee’s dissenting comments from a site that is supposed to be promoting high quality scientific discussions?
The full text of the comment, response and rebuttal was downloaded before removal by one of the readers and can be read here.
Dr. Lee said he is discussing a very serious scientific medical issue. He did not find any inappropriate language in his comments or rebuttals.
Therefore, on behalf of thousands of families around the world dealing with serious new medical conditions after Gardasil administration, the SaneVax team requests that NIH moderators restore the original comment, response and rebuttal to the PubMed Commons’ website.
It is in the public’s best interest that Dr. Schiffman and Dr. Lee continue their scientific debate. Alternatively, the NIH moderators must publicly publish valid reasons for the removal of Dr. Lee’s comments.
In the words of Winston Churchill: In science you don’t need to be polite, you merely have to be right. Open, honest debate is the only way to restore public confidence.
Censorship will not work